When it comes to pharmaceuticals, a label isn’t just a piece of paper, it’s a vital part of the product’s identity, safety, and compliance. A pharmaceutical label communicates critical information that protects both the manufacturer and the end user. But what exactly defines a “pharma label,” and why is it so important in the life sciences industry?
Defining a Pharmaceutical Label
A pharma label is any printed or applied component that identifies a pharmaceutical product and communicates its key information, such as the drug name, dosage, lot number, expiration date, manufacturer, and regulatory data. Beyond aesthetics, pharmaceutical labels play a crucial role in ensuring the accuracy, traceability, and safety of medication throughout its lifecycle from production and packaging to pharmacy shelves and patient use.
The Role of the Label in Compliance
Every pharmaceutical label must meet the rigorous standards set by regulatory authorities like the FDA, EMA, or Health Canada. This means clear legibility, tamper evidence, and durable materials are non-negotiable. Non-compliance can lead to costly recalls, fines, and reputational damage, making precise label production and verification critical steps in pharmaceutical packaging.
Technology and Traceability
Modern pharma labels often incorporate track-and-trace features, such as 2D barcodes, serialization data, or RFID tags. These allow manufacturers and distributors to verify product authenticity and prevent counterfeiting, a major concern in global pharma distribution.

The Bottom Line
Pharmaceutical labels are more than just identifiers; they are the bridge between manufacturers, regulators, and patients. At IL Group, we understand that every label carries life-saving information, and we design our labeling solutions to ensure precision, reliability, and peace of mind in every application.
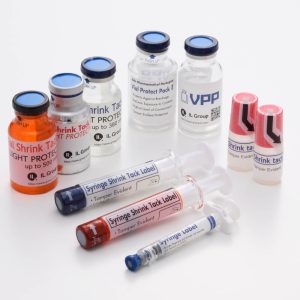
Check out some of our solutions below!
You might also like