Pharmaceutical packaging demands precision, durability, and compliance, and selecting the right label is at the heart of that process. From small vials and blister packs to large infusion bottles, every product has unique labeling needs. Understanding the types of pharmaceutical labels and the factors that influence label selection can help manufacturers improve efficiency, accuracy, and patient safety.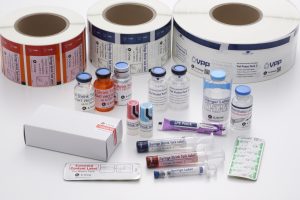 Types of Pharmaceutical Labels
Types of Pharmaceutical Labels
- Primary Labels
These labels appear directly on the drug container, bottles, vials, syringes, or ampoules. They display essential product details such as dosage, active ingredients, and batch number. The materials must withstand sterilization, refrigeration, and chemical exposure. - Secondary Labels
Applied to packaging such as cartons or outer wraps, secondary labels provide supplemental information like barcodes, branding, and regulatory symbols. They often incorporate anti-tampering or security features. - Tamper-Evident Labels
Designed to reveal if a product has been opened or altered, these are vital for protecting product integrity and consumer trust. - Multi-Layer or Peel-and-Read Labels
Used when extensive regulatory or multilingual information is required. These labels save space while ensuring full compliance. - Specialty Labels
Includes temperature-sensitive, RFID-enabled, and serialized labels that support digital tracking and supply chain transparency.
Key Considerations When Selecting Pharma Labels and Labeling Systems
Selecting the right labeling solution involves choosing both the right label and labeling system. The label must have the right materials, functionality, and performance while the labeling system must apply each label with precision and consistency, integrating seamlessly into the packaging line. Below are some key considerations.
- Material Compatibility: Choose label substrates that can handle the storage and sterilization conditions of the drug (e.g., cryogenic temperatures or humidity).
- Adhesive Performance: Medical-grade adhesives must stay secure on a range of surfaces — from glass to plastic — without compromising label integrity.
- Print Durability and Readability: Barcodes, lot numbers, and expiration dates must remain legible throughout the product’s lifecycle.
- Regulatory Compliance: Ensure adherence to standards such as FDA 21 CFR Part 11 and EU FMD serialization requirements.
- Automation Readiness: Labels must be optimized for automated application systems to maintain speed, precision, and repeatability on production lines.

A Label That Works as Hard as Your Product
The right labeling solution enhances efficiency, accuracy, and compliance at every step. At IL Group, we design and manufacture pharmaceutical labels and automated labeling systems that integrate seamlessly with your production process, delivering the precision and reliability your operations demand.
Check out some of our solutions below!
You might also like